 Pussy888 พุซซี่888 ความครบเครื่องของการพนันบนโลกมือถือ สร้างรายได้อย่างใหญ่โต
Pussy888 พุซซี่888 ความครบเครื่องของการพนันบนโลกมือถือ สร้างรายได้อย่างใหญ่โต
Pussy888 เป็นแพลตฟอร์มคาสิโนออนไลน์ที่พรีเซนเทชั่นเกมสล็อตที่นานัปการ เป็นที่นิยมในโลกของการพนันออนไลน์ด้วยเหตุว่าอินเทอร์เฟซที่ใช้งานง่าย การเล่นเกมที่น่าดึงดูด แล้วก็จังหวะที่จะชนะเงินจริง นี่คือเรื่องสำคัญบางประการของสล็อตบน Pussy888 pussy888 มีเกมสล็อตให้เลือกมากมาย ตั้งแต่สล็อตสามวงล้อคลาสสิกไปจนถึงสล็อตวิดีโอยุคใหม่ที่มีช่องชำระเงินหลายช่อง คุณสมบัติโบนัส รวมทั้งธีม ผู้เล่นสามารถค้นหาเกมที่ตรงกับความอยากได้ของพวกเขา ไม่ว่าพวกเขาจะเพลินกับเครื่องผลไม้แบบเริ่มแรกหรือสล็อตรูปแบบใหม่ๆที่สร้างขึ้นมาเพื่อนักเดิมพันทุกกลุ่ม
เล่นสล็อตเข้าถึงง่ายผ่านมือถือ รองรับเครื่องใช้ไม้สอยการพนันทุกรุ่น
Pussy888 พุซซี่888 ได้รับการปรับให้เหมาะสมกับการเล่นเกมบนมือถือ ช่วยทำให้ผู้เล่นเพลิดเพลินเจริญใจไปกับเกมสล็อต ที่ถูกใจบนสมาร์ทโฟนรวมทั้งแท็บเล็ต ความสบายสบายนี้ทำให้เป็นตัวเลือกลำดับแรกๆสำหรับผู้เล่นที่ชื่นชอบในด้านการเล่นเกมระหว่างเดินทาง เพิ่มประสบการณ์ใหม่ให้กับผู้ใช้แพลตฟอร์ม โดยผู้พัฒนาระบบของพวกเรานี้ให้ความเอาใจใส่กับประสบการณ์ผู้ใช้ที่ราบระรื่นและเบิกบาน รูแบบที่ใช้งานง่ายแล้วก็เพิ่มความสะดวกสบายในการใช้งาน ทำให้ทั้งผู้เล่นมือใหม่แล้วก็ผู้เล่นที่มีประสบการณ์ สามารถเข้าถึงได้ การเข้าถึงเกมเดิมพันได้ง่าย และพิจารณาถึงสิ่งที่ดีต่อผู้ใช้นี้มีส่วนทำให้เป็นที่นิยม
โบนัสรวมทั้งโปรโมชั่นคุ้ม พร้อมมอบให้ลูกค้า พุซซี่888 พุซซี่888
เหมือนกันกับคาสิโนออนไลน์หลายแห่ง ที่มีการเสนอโบนัสแล้วก็โปรโมชั่นจำนวนมากเพื่อดึงดูดรักษาผู้เล่น สิ่งพวกนี้อาจรวมทั้งโบนัสต้อนรับ ฟรีสปิน และรางวัลตอบแทนผู้ใช้งาน ทำให้ผู้เล่นมีโอกาสพิเศษที่จะชนะโดยทั่วไปแล้ว พุซซี่888 พุซซี่888 จะให้การช่วยเหลือลูกค้าเพื่อช่วยเหลือผู้เล่นในการไต่ถามหรือปัญหาอะไรก็ตามที่พวกเขาอาจพบขณะเล่นสล็อตหรือใช้แพลตฟอร์ม สิ่งสำคัญเป็นจำเป็นต้องรู้ดีว่าความพร้อมใช้งานรวมทั้งคุณสมบัติของพวกเราบางทีอาจเปลี่ยนแปลงไปตามยุคสมัย เพราะฉะนั้นก็เลยแนะนำให้เยี่ยมชมเว็บอย่างเป็นทางการหรือติดต่อฝ่ายสนับสนุนลูกค้า เพื่อรับข้อมูลล่าสุดเกี่ยวกับเกมสล็อตและก็บริการของเรา นอกจากนั้นพวกเรายังพร้อมให้บริการการเดิมพันด้วยคุณภาพ
ความปลอดภัยและก็การเล่นมีความเป็นธรรม
คาสิโนออนไลน์ที่มีชื่อให้ความใส่ใจกับความปลอดภัยและก็การเล่นที่ชอบธรรม พวกเราใช้เทคโนโลยีการเข้ารหัสเพื่อปกป้องข้อมูลของผู้เล่น และก็รับประกันว่าเกมสล็อตมีความเที่ยงธรรม โดยไม่มีประวัติการโกงซึ่งสามารถวิเคราะห์จากการรีวิว แล้วก็บอกต่อบรอคอยการจากผู้ใช้งานจริงได้เลย ทั้งตัวเลือกการจ่ายเงินโดยทั่วไปเรามีวิธีการชำระเงินที่นานัปการ สำหรับเพื่อการฝากรวมทั้งถอนเงิน รวมทั้งบัตรเครดิต กระเป๋าเงินอิเล็กทรอนิกส์ แล้วก็การโอนเงินผ่านแบงค์ ความยืดหยุ่นนี้ทำให้ผู้เล่นสามารถจัดแจงเงินลงทุนของตัวเองได้สบาย
เพราะเหตุไรเกมสล็อต pussy888 ทั้งในระบบทั่วไปแล้วก็ออนไลน์ถึงได้รับความนิยมอย่างมาก
แน่ๆ! ในบริบทของการเล่นเกม โดยธรรมดา สล็อต จะหมายถึงเกมคาสิโนประเภทหนึ่งที่เรียกว่า
สล็อตออนไลน์ นี่คือหัวข้อสำคัญบางประการเกี่ยวกับสล็อตเกมจากค่าย pussy888fun
รากฐานของสล็อตออนไลน์ สล็อตออนไลน์เป็นเกมการเดิมพันที่ได้รับความนิยมที่เจอได้ทั้งในคาสิโนทั่วๆไปรวมทั้งคาสิโนออนไลน์จะมีวงล้อ (ธรรมดาสามวงขึ้นไป) ที่หมุนเมื่อผู้เล่นเปิดใช้งาน
• สัญลักษณ์สล็อตออนไลน์ pussy888 มีสัญลักษณ์ต่างๆบนวงล้อ เป็นต้นว่า ผลไม้ ตัวเลข ตัวเขียน และไอคอนตามธีม ชุดค่าผสมที่ชนะจะเกิดขึ้นเมื่อสัญลักษณ์เฉพาะเรียงกันในลักษณะใดลักษณะหนึ่ง
• ช่องชำระเงิน ช่องจ่ายเงินเป็นเส้นที่พาดผ่านวงล้อซึ่งสามารถกำเนิดชุดค่าผสมที่ชนะได้ สล็อตออนไลน์แต่ละเครื่องมีจำนวนช่องจ่ายเงินที่ต่างกัน ตั้งแต่บรรทัดเดียวไปจนกระทั่งหลักร้อยหรือหลักพัน
• การเดิมพันและการจ่ายเงินผู้เล่นวางเดิมพันก่อนหมุนวงล้อ และจำนวนเงินที่ชนะจะขึ้นกับการรวมกันของสัญลักษณ์เฉพาะที่ปรากฏบนเพย์ไลน์ การชำระเงินบางทีอาจแตกต่างอย่างยิ่ง โดยบางสล็อตเสนอแจ็คพอตแบบโปรเกรสซีฟที่อาจจะก่อให้กำเนิดความมีชัยครั้งใหญ่
• ธีมแล้วก็ฟีพบร์ สล็อตออนไลน์มีธีมมากมาย ได้แก่ อียิปต์โบราณ ตำนาน ภาพยนตร์ รวมทั้งอื่นๆอีกมากมาย สล็อตจำนวนมากยังมีฟีพบร์โบนัส ตัวอย่างเช่น ฟรีสปิน เครื่องหมายเสริม และมินิเกมเพื่อทำให้การเล่นเกมน่าตื่นเต้นยิ่งขึ้น
• ตัวสร้างเนื้อสร้างตัวเลขสุ่ม (RNG) สล็อตออนไลน์ยุคใหม่ใช้เทคโนโลยี RNG เพื่อแน่ใจว่าคำตอบของการหมุนแต่ละครั้งจะเป็นแบบสุ่มแล้วก็เป็นกลางทั้งหมด ซึ่งหมายความว่าการชนะเป็นเรื่องของโชคเป็นส่วนมาก
สล็อตออนไลน์ สล็อต ปากทางเข้าเล่นเสถียรภาพ ระบบมั่นคง
เกมสล็อตจึงเข้าถึงได้ง่ายยิ่งขึ้น ผู้เล่นสามารถเพลิดเพลินกับสล็อตที่มากมายได้จากที่บ้านหรือบนเครื่องไม้เครื่องมือโทรศัพท์มือถือ เดิมพันอย่างมีสติ สล็อตออนไลน์ pussy888 เป็นความหรรษา แต่ว่าก็สามารถเสพติดได้เช่นเดียวกัน เป็นสิ่งจำเป็นสำหรับผู้เล่นในการพนันอย่างมีความรับผิดชอบรวมทั้งระบุวงเงินการใช้จ่ายของพวกเขา สล็อตออนไลน์เป็นเยี่ยมในเกมคาสิโนยอดนิยมสูงที่สุดเพราะเหตุว่าความเรียบง่ายและมีโอกาสจ่ายเงินรางวัลจำนวนไม่น้อย ผู้เล่นหลากหลายประเภทชื่นชอบตั้งแต่นักพนันทั่วๆไปไปจนถึงนักเดิมพันระดับที่ถือว่าสูง โปรดจดจำไว้ว่าในช่วงเวลาที่การเล่นสล็อตสามารถครึกครื้นรวมทั้งสร้างกำไรได้ พุซซี่888 แม้กระนั้นก็มีความเสี่ยงสำหรับเพื่อการสูญเสียเงินเหมือนกัน สิ่งจำเป็นเป็นต้องเล่นการพนันอย่างมีมีสติและอยู่ในทางกำหนดของคุณ
การลงเดิมพันเกมสล็อตออนไลน์ สล็อต บนเว็บแห่งนี้ เป็นอีกหนึ่งหนทางแนวทางการทำเงินสร้างรายได้ที่รื้นเริง แล้วก็พร้อมแจกโบนัสรางวัล ยินดีต้อนรับนักพนันที่มีทุนน้อย ให้ได้สัมผัสกับเว็บตรงไม่ผ่านเอเย่นต์ ที่สามารถเข้าถึงได้ผ่านทางหน้าเว็บ หรือจะติดตั้งแอปพลิเคชันลงบนมือถือก็สามารถทำเป็น
ค่าย พุซซี่888 pussy888 บริการ 24 ชั่วโมง 2 SEP 23 Frances พนัน สล็อตรางวัลแจ็คพอต Top 27
ขอขอบคุณby พุซซี่888



 วันนี้เรามาบอกสูตรหรือเทคนิคดีๆที่จะแปลงนักเดิมพันมือใหม่ ให้เปลี่ยนเป็นผีพนันบอล
วันนี้เรามาบอกสูตรหรือเทคนิคดีๆที่จะแปลงนักเดิมพันมือใหม่ ให้เปลี่ยนเป็นผีพนันบอล  เลือกเดินพันสล็อตออนไลน์กับเว็บของพวกเรา ยืนยันการพนันบอลหลายแบบ ไม่ว่าจะเป็นพนันบอลโดดเดี่ยว แทงบอลเต็ง คาสิโนสด แล้วก็ยังมีแนวทางการเล่น การสอนเทคนิค บอกสูตรต่างๆเหมาะกับนักเสี่ยงดวงมือใหม่ แบบอย่างการเล่นไม่ยาก เพราะเหตุว่าพวกเรามีข้อมูลให้ได้ศึกษาการพนันบอลต่างๆไว้ให้ละเอียดครบถ้วน เล่นเว็บพวกเราสามารถแทงได้ทุกคนอย่างแน่นอน
เลือกเดินพันสล็อตออนไลน์กับเว็บของพวกเรา ยืนยันการพนันบอลหลายแบบ ไม่ว่าจะเป็นพนันบอลโดดเดี่ยว แทงบอลเต็ง คาสิโนสด แล้วก็ยังมีแนวทางการเล่น การสอนเทคนิค บอกสูตรต่างๆเหมาะกับนักเสี่ยงดวงมือใหม่ แบบอย่างการเล่นไม่ยาก เพราะเหตุว่าพวกเรามีข้อมูลให้ได้ศึกษาการพนันบอลต่างๆไว้ให้ละเอียดครบถ้วน เล่นเว็บพวกเราสามารถแทงได้ทุกคนอย่างแน่นอน 3. สูตรแทงสูงท้ายเกม การพนันบอลจะสามารถเลือกได้ว่าจะแทงเฉพาะตอนครึ่งแรก หรือแทงแบบเต็มเวลา ทำให้นักเดิมพันสามารถดูโอกาสสำหรับเพื่อการทำประตูของการประลองนี้ได้ โดยสูตรนี้จะให้นักพนันรอเวลาจนกระทั่งตอนใกล้่จบเกม หรือช่วง 25 นาทีสุดท้าย ถ้ายังมีสกอร์ หรือได้โอกาสน้อยที่จะทำคะแนน การแทงสูง ก็จะได้โอกาสชนะมากกว่านั่นเอง
3. สูตรแทงสูงท้ายเกม การพนันบอลจะสามารถเลือกได้ว่าจะแทงเฉพาะตอนครึ่งแรก หรือแทงแบบเต็มเวลา ทำให้นักเดิมพันสามารถดูโอกาสสำหรับเพื่อการทำประตูของการประลองนี้ได้ โดยสูตรนี้จะให้นักพนันรอเวลาจนกระทั่งตอนใกล้่จบเกม หรือช่วง 25 นาทีสุดท้าย ถ้ายังมีสกอร์ หรือได้โอกาสน้อยที่จะทำคะแนน การแทงสูง ก็จะได้โอกาสชนะมากกว่านั่นเอง
 |
|


 2. มีคู่มือการสร้างคิวอาร์รหัสที่ละเอียดมาก สร้าง qr code
2. มีคู่มือการสร้างคิวอาร์รหัสที่ละเอียดมาก สร้าง qr code เป็นยังไงบ้างนะครับกับ เพราะเหตุใดจะต้องสร้าง QR Code กับ qrcode.in.th ผมบอกเลยว่า การผลิตคิวอาร์รหัสสำหรับหลายๆคนบางทีอาจจะเกิดเรื่องยาก ทางเราในฐานะ QR Code Generator สร้างคิวอาร์โค้ด จึงพยายามที่จะทำให้การใช้งานทุกๆส่วนนั้นง่ายที่สุด ถ้าเกิดไม่เข้าใจก็จะอธิบายอย่างมาก เพื่อทุกคนสามารถสร้างคิวอาร์โค้ดของตนได้นั่นเองขอรับ ถ้าคนใดพึงพอใจอยากทดลองสร้างคิวอาร์รหัสก็แวะมาใช้บริการกับเราได้เลยจ้ะครับผม สำหรับวันนี้ ผมจำเป็นต้องขอตัวลาไปก่อน แล้วพบกันใหม่ในบทความหน้าครับผม สวัสดีขอรับ
เป็นยังไงบ้างนะครับกับ เพราะเหตุใดจะต้องสร้าง QR Code กับ qrcode.in.th ผมบอกเลยว่า การผลิตคิวอาร์รหัสสำหรับหลายๆคนบางทีอาจจะเกิดเรื่องยาก ทางเราในฐานะ QR Code Generator สร้างคิวอาร์โค้ด จึงพยายามที่จะทำให้การใช้งานทุกๆส่วนนั้นง่ายที่สุด ถ้าเกิดไม่เข้าใจก็จะอธิบายอย่างมาก เพื่อทุกคนสามารถสร้างคิวอาร์โค้ดของตนได้นั่นเองขอรับ ถ้าคนใดพึงพอใจอยากทดลองสร้างคิวอาร์รหัสก็แวะมาใช้บริการกับเราได้เลยจ้ะครับผม สำหรับวันนี้ ผมจำเป็นต้องขอตัวลาไปก่อน แล้วพบกันใหม่ในบทความหน้าครับผม สวัสดีขอรับ ขอขอบพระคุณreference
ขอขอบพระคุณreference 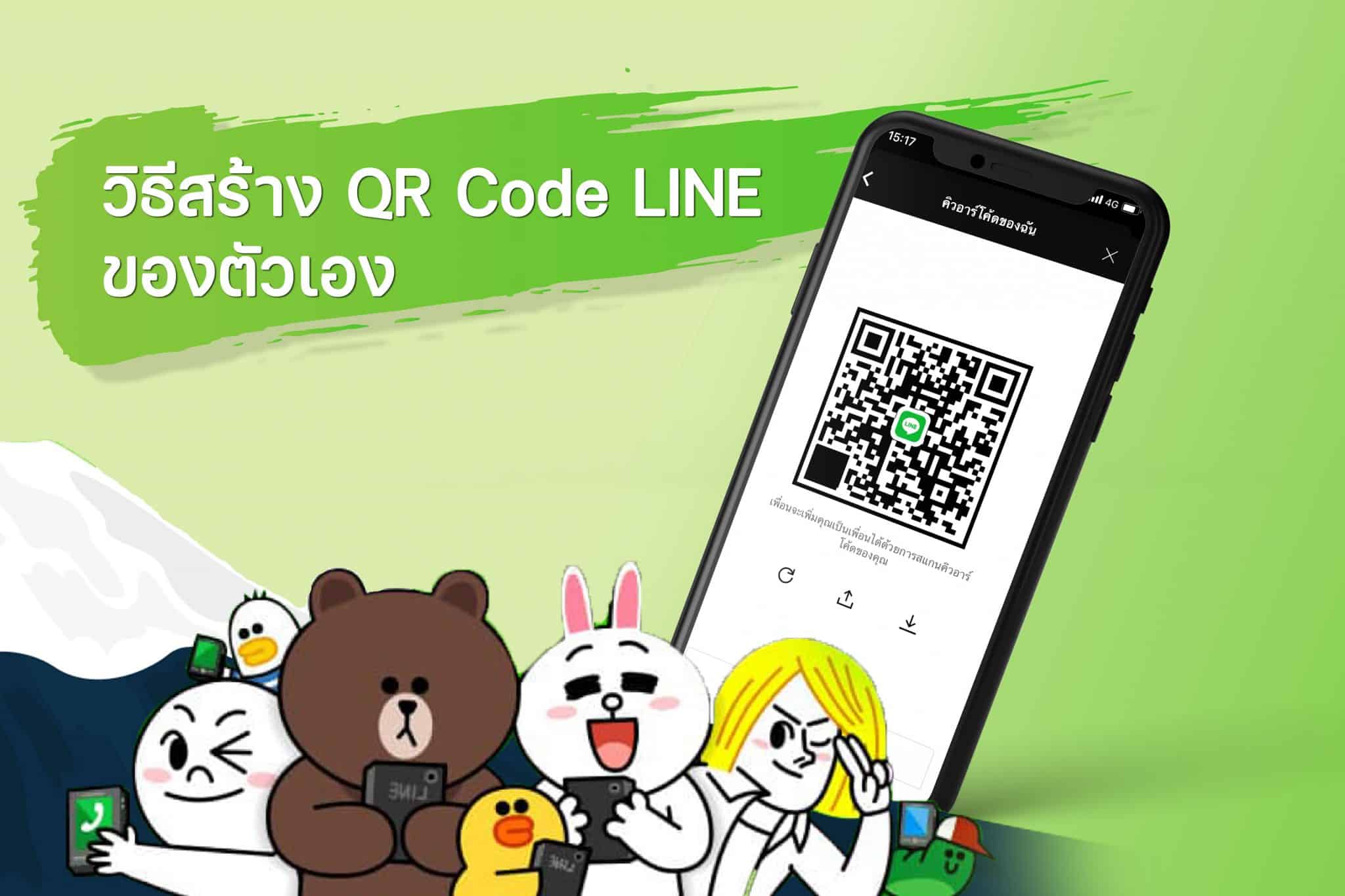
 slotxo
slotxo  เล่นเกมสล็อตออนไลน์สุดคลาสสิค จุดเริ่มของความสนุกสนานร่าเริง
เล่นเกมสล็อตออนไลน์สุดคลาสสิค จุดเริ่มของความสนุกสนานร่าเริง
 ในวันนี้เป็นจังหวะอันดีที่เว็บไซต์ ดูหนังใหม่ ดูหนังออนไลน์ 2023 ดูหนังผ่านเน็ต 2023 หนังออนไลน์ 2566 ของพวกเรา movieskub จะได้เฉิดฉันแสงกันแบบมุทะลุ พร้อมหนังงใหม่ชนโรงประสิทธิภาพหนังโรงแบบจัดหนักจัดเต็มกันเลยทีเดียว ประเดี๋ยวพวกเรา เว็บไซต์ ดูหนังใหม่ ดูหนังผ่านอินเตอร์เน็ต 2023 หนังออนไลน์ 2566 movieskub จะบอกคุณสมบัติของเว็บไซต์ ดูหนังใหม่ ดูหนังออนไลน์ 2023 หนังออนไลน์ 2566 ที่ดีให้กับทุกท่านได้รับทราบกันอย่างทั่วถึงเองจ้า บอกเลยว่า ที่พวกเราจะบอกทุกคนไปทั้งหมดนี่ เว็บ ดูหนังใหม่ ดูหนังออนไลน์ 2023 หนังออนไลน์ 2566 ของพวกเรา movieskub มีครบจบในที่เดียวเลยน้า เริ่มเลอ
ในวันนี้เป็นจังหวะอันดีที่เว็บไซต์ ดูหนังใหม่ ดูหนังออนไลน์ 2023 ดูหนังผ่านเน็ต 2023 หนังออนไลน์ 2566 ของพวกเรา movieskub จะได้เฉิดฉันแสงกันแบบมุทะลุ พร้อมหนังงใหม่ชนโรงประสิทธิภาพหนังโรงแบบจัดหนักจัดเต็มกันเลยทีเดียว ประเดี๋ยวพวกเรา เว็บไซต์ ดูหนังใหม่ ดูหนังผ่านอินเตอร์เน็ต 2023 หนังออนไลน์ 2566 movieskub จะบอกคุณสมบัติของเว็บไซต์ ดูหนังใหม่ ดูหนังออนไลน์ 2023 หนังออนไลน์ 2566 ที่ดีให้กับทุกท่านได้รับทราบกันอย่างทั่วถึงเองจ้า บอกเลยว่า ที่พวกเราจะบอกทุกคนไปทั้งหมดนี่ เว็บ ดูหนังใหม่ ดูหนังออนไลน์ 2023 หนังออนไลน์ 2566 ของพวกเรา movieskub มีครบจบในที่เดียวเลยน้า เริ่มเลอ 1. โปรโมทไม่เกะกะดวงตา บอกเลยข้อนี้ ดูหนังใหม่ ใครกันแน่ก็อยากได้ ถ้าไม่ได้ดูในแอพเสียตังนะ ไม่มีทางจะได้แบบเว็บของเรา เพราะพวกเราจัดแจงให้โฆษณาอยู่ในที่ที่ควรอยู่ ทุกท่านอยากมองอะไรกดมองได้เอง โดยที่โปรโมทในเว็บไซต์
1. โปรโมทไม่เกะกะดวงตา บอกเลยข้อนี้ ดูหนังใหม่ ใครกันแน่ก็อยากได้ ถ้าไม่ได้ดูในแอพเสียตังนะ ไม่มีทางจะได้แบบเว็บของเรา เพราะพวกเราจัดแจงให้โฆษณาอยู่ในที่ที่ควรอยู่ ทุกท่านอยากมองอะไรกดมองได้เอง โดยที่โปรโมทในเว็บไซต์ 

 สมาชิกใหม่รับ 50% จาก 918VIP
สมาชิกใหม่รับ 50% จาก 918VIP สวัสดีครับชาว 918kiss download ทุกคน ในตอนอาทิตย์ที่แล้ว ทางพวกเราได้มองเห็นปัญหาที่เกี่ยวข้องกับการแบน URL ของผู้ให้บริการอินเตอร์เน็ตหลายๆค่าย ไม่ว่าจะเป็นทรู ดีแทค หรือเอไอเอส เรียกว่าแบนกันซ้ำๆ ทางเข้า
สวัสดีครับชาว 918kiss download ทุกคน ในตอนอาทิตย์ที่แล้ว ทางพวกเราได้มองเห็นปัญหาที่เกี่ยวข้องกับการแบน URL ของผู้ให้บริการอินเตอร์เน็ตหลายๆค่าย ไม่ว่าจะเป็นทรู ดีแทค หรือเอไอเอส เรียกว่าแบนกันซ้ำๆ ทางเข้า 

 บาคาร่าเว็บไหนดี บาคาร่าออนไลน์ www.Sexybaccarat168.com 10 กันยา 2565 เว็บไซต์เด็ด เว็บดัง ปังวัวรตๆมาที่นี่รวยแน่แค่เล่นบาคาร่า
บาคาร่าเว็บไหนดี บาคาร่าออนไลน์ www.Sexybaccarat168.com 10 กันยา 2565 เว็บไซต์เด็ด เว็บดัง ปังวัวรตๆมาที่นี่รวยแน่แค่เล่นบาคาร่า ในยุคที่เทคโนโลยีเริ่มเข้ามามีหน้าที่มากขึ้นเรื่อยๆในชีวิตประจำวันของพวกเรานั้น ความสบายสบาย ความรวดเร็ว รวมทั้งความนำสมัยต่างๆก็มีเยอะขึ้นเรื่อยๆ นำมาซึ่งการทำให้เทคโนโลยีหรือของใหม่เก่าๆอะไรบางอย่างนั้นถูกแทนที่ ปรับปรุง หรือหายไปเลยก็ว่าได้ครับผม อาทิเช่น การเข้ามาของแฟลชไดรฟ์ที่ตอบแทนฟลอปปี้ดิสก์หรือที่เราเรียกกันอย่างชินปากว่า แผ่นดิสก์
ในยุคที่เทคโนโลยีเริ่มเข้ามามีหน้าที่มากขึ้นเรื่อยๆในชีวิตประจำวันของพวกเรานั้น ความสบายสบาย ความรวดเร็ว รวมทั้งความนำสมัยต่างๆก็มีเยอะขึ้นเรื่อยๆ นำมาซึ่งการทำให้เทคโนโลยีหรือของใหม่เก่าๆอะไรบางอย่างนั้นถูกแทนที่ ปรับปรุง หรือหายไปเลยก็ว่าได้ครับผม อาทิเช่น การเข้ามาของแฟลชไดรฟ์ที่ตอบแทนฟลอปปี้ดิสก์หรือที่เราเรียกกันอย่างชินปากว่า แผ่นดิสก์ เริ่มเล่นบาคาร่า ทีแรกก็ช่วยคุณได้แน่นอน เชื่อสิคุ้มค่ามากๆ
เริ่มเล่นบาคาร่า ทีแรกก็ช่วยคุณได้แน่นอน เชื่อสิคุ้มค่ามากๆ ถ้าเกิดผู้ใดกันต้องการจะทดสอบเล่นแอปฯ สมัครบาคาร่า ที่แตกต่างของแต่ละคน บางบุคคลต้องการจจะเล่น เพื่อเอาความสนุก ทุเลาสมองที่เหนื่อย จากการทำงานมาทั้งวัน หรือบางคนเล่นเพื่อเอาเงินรางวัลจากมัน ไปใช้จ่ายในชีวิตประจำวัน แต่ว่าไม่ว่าจะเหตุผลอะไรก็ตามมแต่ ขั้นตอนเบื้องต้นพวกเราจึงต้องควรทราบไว้แล้วก็กระทำตามอย่างเดียวกันหมดเป็นหาเว็บไซต์ สมัคร sexyauto168.com ได้เลย
ถ้าเกิดผู้ใดกันต้องการจะทดสอบเล่นแอปฯ สมัครบาคาร่า ที่แตกต่างของแต่ละคน บางบุคคลต้องการจจะเล่น เพื่อเอาความสนุก ทุเลาสมองที่เหนื่อย จากการทำงานมาทั้งวัน หรือบางคนเล่นเพื่อเอาเงินรางวัลจากมัน ไปใช้จ่ายในชีวิตประจำวัน แต่ว่าไม่ว่าจะเหตุผลอะไรก็ตามมแต่ ขั้นตอนเบื้องต้นพวกเราจึงต้องควรทราบไว้แล้วก็กระทำตามอย่างเดียวกันหมดเป็นหาเว็บไซต์ สมัคร sexyauto168.com ได้เลย หารายได้ได้มาก ช่วยคุณได้ง่ายๆ
หารายได้ได้มาก ช่วยคุณได้ง่ายๆ ขอขอบคุณมากอ้างอิงจาก
ขอขอบคุณมากอ้างอิงจาก 
 เว็บไซต์สล็อตที่มาแรงเวลานี้ ที่ดีไม่ซ้ำใคร คุ้มสุดๆ
เว็บไซต์สล็อตที่มาแรงเวลานี้ ที่ดีไม่ซ้ำใคร คุ้มสุดๆ เว็บเด็ด ระดับโลก เชื่อเลยว่าคุ้มและก็ดี
เว็บเด็ด ระดับโลก เชื่อเลยว่าคุ้มและก็ดี ขอขอบคุณby web
ขอขอบคุณby web  สวัสดีครับผมชาว m.hengjing168.win สล็อต ทุกคน ขณะนี้เป็นอย่างไรกันบ้างครับ? ผมบอกเลยว่า ขณะนี้นอกเหนือจากเรื่องสล็อตออนไลน์ที่ผมพึงพอใจเป็นพิเศษอยู่แล้ว ผมกำลังพึงพอใจเรื่องของสภาพภูมิอากาศครับ หนาวมาก! เวลาที่ผมเขียนเนื้อหาของบทความนี้อยู่ เชื่อไหมครับว่าผมใส่เสื้อตั้ง 2 ตัวเลยจ๊าครับผม แล้วนี่ม.ค.นะครับ! นอกเหนือจากอากาศผันแปรแล้ว ฝุ่นละอองก็จำนวนมากด้วย ทุกคนก็ดูแลสุขภาพกันด้วยครับผม นอนเล่น 168สล็อต กันชิวๆอยู่บ้านดีมากยิ่งกว่าออกไปด้านนอกบ้านครับผมช่วงนี้ ฮ่า… สล็อต168
สวัสดีครับผมชาว m.hengjing168.win สล็อต ทุกคน ขณะนี้เป็นอย่างไรกันบ้างครับ? ผมบอกเลยว่า ขณะนี้นอกเหนือจากเรื่องสล็อตออนไลน์ที่ผมพึงพอใจเป็นพิเศษอยู่แล้ว ผมกำลังพึงพอใจเรื่องของสภาพภูมิอากาศครับ หนาวมาก! เวลาที่ผมเขียนเนื้อหาของบทความนี้อยู่ เชื่อไหมครับว่าผมใส่เสื้อตั้ง 2 ตัวเลยจ๊าครับผม แล้วนี่ม.ค.นะครับ! นอกเหนือจากอากาศผันแปรแล้ว ฝุ่นละอองก็จำนวนมากด้วย ทุกคนก็ดูแลสุขภาพกันด้วยครับผม นอนเล่น 168สล็อต กันชิวๆอยู่บ้านดีมากยิ่งกว่าออกไปด้านนอกบ้านครับผมช่วงนี้ ฮ่า… สล็อต168

 เล่นสล็อตจำเป็นต้องที่นี่ PG
เล่นสล็อตจำเป็นต้องที่นี่ PG  1. สมัครฟรี ไร้ค่าจารีต ทุกคนสมาชิกท่านใดพึงพอใจเดิมพันเกมสล็อตออนไลน์ ฟีเจอร์จ่ายกำไรให้มากที่สุดได้กำไรจริงโดยการเติมถอนผ่านทรูมันนี่ ท่านก็สามารถสมัครใช้งานได้ฟรีไม่จำเป็นต้องเสียค่าใช้จ่ายในการสมัครหรือค่าธรรมเนียมแรกเข้าอีกด้วยนะคะ
1. สมัครฟรี ไร้ค่าจารีต ทุกคนสมาชิกท่านใดพึงพอใจเดิมพันเกมสล็อตออนไลน์ ฟีเจอร์จ่ายกำไรให้มากที่สุดได้กำไรจริงโดยการเติมถอนผ่านทรูมันนี่ ท่านก็สามารถสมัครใช้งานได้ฟรีไม่จำเป็นต้องเสียค่าใช้จ่ายในการสมัครหรือค่าธรรมเนียมแรกเข้าอีกด้วยนะคะ







 รูปแบบการใช้งานของ jubyet69 หนัง 18 ฟรีมีระบบระเบียบอะไรที่เป็นประโยชน์กับทุกคนบ้าง?
รูปแบบการใช้งานของ jubyet69 หนัง 18 ฟรีมีระบบระเบียบอะไรที่เป็นประโยชน์กับทุกคนบ้าง?


 2. ระบุเงินลงทุนในแต่ละครั้งให้แน่ชัด
2. ระบุเงินลงทุนในแต่ละครั้งให้แน่ชัด 3. หาขณะที่เหมาะสมกับตนเองในการเล่นพีจีสล็อต
3. หาขณะที่เหมาะสมกับตนเองในการเล่นพีจีสล็อต
 เล่นสล็อตออนไลน์ ทำไมจำเป็นต้องรำลึกถึง jinda888 สล็อตเว็บตรงไม่ผ่านเอเย่นต์
เล่นสล็อตออนไลน์ ทำไมจำเป็นต้องรำลึกถึง jinda888 สล็อตเว็บตรงไม่ผ่านเอเย่นต์ ลงทะเบียนเป็นสมาชิกรับโบนัส ได้ทันทีหลังจากสมัคร
ลงทะเบียนเป็นสมาชิกรับโบนัส ได้ทันทีหลังจากสมัคร
 รีวิว ธุรกิจการบิน ม.ราชภัฏ
รีวิว ธุรกิจการบิน ม.ราชภัฏ ตรงนี้ ก็เลยค่อนข้างจะให้ความใส่ใจมากมายก่ายกอง ในเรื่องเกี่ยวกับการใช้ภาษาหรือรวมไปถึง การจัดการในระดับประเทศ ที่สามารถเรียกได้เลยว่ามีการขนเอาหลักสูตรต่างๆในระดับสากลมาเอาไว้ในที่นี้ที่เดียว อย่างชัดเจนรวมทั้งเป็นอะไรที่ค่อนข้างล้ำหน้าไปๆมาๆกเลย สำหรับเพื่อการทำความเข้าใจจาก สวนสุนันทา ที่เปิดสอนถึง 4 วิชาสาขาอย่างยิ่งจริงๆอาทิเช่น
ตรงนี้ ก็เลยค่อนข้างจะให้ความใส่ใจมากมายก่ายกอง ในเรื่องเกี่ยวกับการใช้ภาษาหรือรวมไปถึง การจัดการในระดับประเทศ ที่สามารถเรียกได้เลยว่ามีการขนเอาหลักสูตรต่างๆในระดับสากลมาเอาไว้ในที่นี้ที่เดียว อย่างชัดเจนรวมทั้งเป็นอะไรที่ค่อนข้างล้ำหน้าไปๆมาๆกเลย สำหรับเพื่อการทำความเข้าใจจาก สวนสุนันทา ที่เปิดสอนถึง 4 วิชาสาขาอย่างยิ่งจริงๆอาทิเช่น

 ดูหนังx เว็บไซต์หนังอาร์คลิปมาแรงจำต้อง
ดูหนังx เว็บไซต์หนังอาร์คลิปมาแรงจำต้อง  เว็บหนังโป๊ jubyet69 ดูหนังxของเราจะก่อให้ท่านมีความสุข รู้สึกไม่มีอันตรายวางใจเราได้ ทุกท่านจะสัมผัสได้ถึงความตื่นเต้นและก็น่าสนใจที่เว็บไซต์ของพวกเรามีให้ทุกครั้งเมื่อไรที่ท่านเข้ามามองเว็บของเรา แล้วท่านจะรู้สึกชื่นชอบใจรวมทั้งรู้สึกไม่มีอันตรายทุกคราวและก็สิ่งที่จำเป็นที่สุด ซึ่งก็คือ การที่ท่านสามารถแฮปปี้กับเว็บของเราได้ในทุกๆครั้ง รวมทั้งสามารถเชื่อใจเราได้เสมอ เราจะไม่มีโปรโมทล้นหลามมาคั่นจังหวะการดูวิดีโอมาให้แก่คุณกวนประสาทโดยเด็ดขาด ท่านสามารถเลือกมองเรื่องเอวีซับไทยได้ เรามีให้เลือกดู คนประเทศไทยกับเอวีซับไทยเป็นของคู่กันจริงล่ะมั้ยขา
เว็บหนังโป๊ jubyet69 ดูหนังxของเราจะก่อให้ท่านมีความสุข รู้สึกไม่มีอันตรายวางใจเราได้ ทุกท่านจะสัมผัสได้ถึงความตื่นเต้นและก็น่าสนใจที่เว็บไซต์ของพวกเรามีให้ทุกครั้งเมื่อไรที่ท่านเข้ามามองเว็บของเรา แล้วท่านจะรู้สึกชื่นชอบใจรวมทั้งรู้สึกไม่มีอันตรายทุกคราวและก็สิ่งที่จำเป็นที่สุด ซึ่งก็คือ การที่ท่านสามารถแฮปปี้กับเว็บของเราได้ในทุกๆครั้ง รวมทั้งสามารถเชื่อใจเราได้เสมอ เราจะไม่มีโปรโมทล้นหลามมาคั่นจังหวะการดูวิดีโอมาให้แก่คุณกวนประสาทโดยเด็ดขาด ท่านสามารถเลือกมองเรื่องเอวีซับไทยได้ เรามีให้เลือกดู คนประเทศไทยกับเอวีซับไทยเป็นของคู่กันจริงล่ะมั้ยขา เล่นบาคาร่าอย่างไรให้ปัง บาคาร่า168 เกมไพ่คลาสสิกต้นตำหรับของการเดิมพันออนไลน์!
เล่นบาคาร่าอย่างไรให้ปัง บาคาร่า168 เกมไพ่คลาสสิกต้นตำหรับของการเดิมพันออนไลน์! ขอขอบคุณมากby
ขอขอบคุณมากby 

 ทำไมจำต้องเล่นสล็อตเว็บไซต์ตรง สล็อต
ทำไมจำต้องเล่นสล็อตเว็บไซต์ตรง สล็อต



 movie2k
movie2k  ซีรี่ย์จีน ย้อนยุค ดูหนังออนไลน์ 2023 Movie2k.io 7 Apr 66 Cassandra สารคดี
ซีรี่ย์จีน ย้อนยุค ดูหนังออนไลน์ 2023 Movie2k.io 7 Apr 66 Cassandra สารคดี 
 มาดูหนัง GDH ที่ madoohd.com กันเหอะ EP.2
มาดูหนัง GDH ที่ madoohd.com กันเหอะ EP.2 สวัสดีครับชาว ดูหนัง ทุกท่าน เป็นยังไงบ้างครับ กลับมาเจอกับผมแล้วก็บทความเว็บไซต์ดูหนังออนไลน์กับ มาดูหนัง GDH ที่ madoohd.com กันเถอะ EP.2 แล้วนะครับ ใน EP.1 ผมได้แนะนำให้ทุกคนไปแล้ว 5 เรื่องร่วมกัน
สวัสดีครับชาว ดูหนัง ทุกท่าน เป็นยังไงบ้างครับ กลับมาเจอกับผมแล้วก็บทความเว็บไซต์ดูหนังออนไลน์กับ มาดูหนัง GDH ที่ madoohd.com กันเถอะ EP.2 แล้วนะครับ ใน EP.1 ผมได้แนะนำให้ทุกคนไปแล้ว 5 เรื่องร่วมกัน เป็นยังไงบ้างขอรับกับ มาดูหนัง GDH ที่ ดูหนังออนไลน์ กันเถิด EP.2 ผมบอกเลยว่า การมาดูหนังชนโรงหรือดูหนังรวมทั้งซีรีส์ที่เว็บไซต์ดูหนังผ่านอินเตอร์เน็ตของเรานั้นแฮปปี้และเอนจอยแน่นอนครับ เนื่องจากว่าคุณจะได้รับประสบการณ์การดูหนังแบบ Full HD เลยล่ะนะครับ ทั้งภาพและก็เสียงเป็นดีแบบสุดๆซึ่งใครที่เลือกหนังไม่ได้ก็นี่เลยครับ หนัง GDH ที่ผมเขียนเอาไว้ให้ทั้งยัง 2 EP. สนใจเรื่องไหนก็จิ้มได้เลยขอรับ สำหรับวันนี้ ผมก็ต้องขอตัวลาไปก่อน แล้วเจอกันใหม่บทความหน้าครับ สวัสดีขอรับ
เป็นยังไงบ้างขอรับกับ มาดูหนัง GDH ที่ ดูหนังออนไลน์ กันเถิด EP.2 ผมบอกเลยว่า การมาดูหนังชนโรงหรือดูหนังรวมทั้งซีรีส์ที่เว็บไซต์ดูหนังผ่านอินเตอร์เน็ตของเรานั้นแฮปปี้และเอนจอยแน่นอนครับ เนื่องจากว่าคุณจะได้รับประสบการณ์การดูหนังแบบ Full HD เลยล่ะนะครับ ทั้งภาพและก็เสียงเป็นดีแบบสุดๆซึ่งใครที่เลือกหนังไม่ได้ก็นี่เลยครับ หนัง GDH ที่ผมเขียนเอาไว้ให้ทั้งยัง 2 EP. สนใจเรื่องไหนก็จิ้มได้เลยขอรับ สำหรับวันนี้ ผมก็ต้องขอตัวลาไปก่อน แล้วเจอกันใหม่บทความหน้าครับ สวัสดีขอรับ
 • pg ศึกษาเล่าเรียนเว็บไซต์สล็อตออนไลน์ที่จะเล่นก่อน ในยุคนี้ คนไหนกันไม่เล่นสล็อตเว็บไซต์ตรงจัดว่าคุณพลาดแล้วนะครับ เนื่องจากว่าการเล่นสล็อตออนไลน์ที่เป็นเว็บตรงอย่างสล็อตpgแท้นั้นปลอดภัยมากมายๆครับ คุณจะไม่ต้องวิตกกังวลว่า เล่นไปแล้วจะโดนล็อกผลไหม เล่นได้กำไรแล้วจะถอนได้ไหม ปัญหาเหล่านี้จะไม่เกิดขึ้นกับสล็อตเว็บไซต์ตรงแน่ๆขอรับ
• pg ศึกษาเล่าเรียนเว็บไซต์สล็อตออนไลน์ที่จะเล่นก่อน ในยุคนี้ คนไหนกันไม่เล่นสล็อตเว็บไซต์ตรงจัดว่าคุณพลาดแล้วนะครับ เนื่องจากว่าการเล่นสล็อตออนไลน์ที่เป็นเว็บตรงอย่างสล็อตpgแท้นั้นปลอดภัยมากมายๆครับ คุณจะไม่ต้องวิตกกังวลว่า เล่นไปแล้วจะโดนล็อกผลไหม เล่นได้กำไรแล้วจะถอนได้ไหม ปัญหาเหล่านี้จะไม่เกิดขึ้นกับสล็อตเว็บไซต์ตรงแน่ๆขอรับ

 สำหรับคอหนังที่ถูกใจดูหนังดังบน Netflix ทางเว็บดูหนังออนไลน์ของพวกเราก็อัพเดทมาให้แก่คุณได้ดูหนังออนไลน์อย่างรวดเร็ว ท่านไม่มีความจำเป็นที่จะต้องเสียค่าใช้จ่ายสำหรับการสมัครสมาชิกรายเดือนอีกต่อไป ทางเว็บไซต์ของพวกเรานำหนังใหม่ชนโรง รวมทั้งหนังตามที่มีคุณภาพมาให้ทุกท่านได้รับดูกัน พูดได้ว่าไม่แพ้หนังบนเน็ตฟริกซ์อย่างยิ่งจริงๆ แล้วก็ที่สำคัญเรามีหนังให้เลือกรับดูมากกว่าไม่ว่าจะในกระแส หรือนอกกระแส เชื่อว่าทุกคนจะได้รับความสนุกสนานแบบเต็มอิ่ม ครบทุกอรรถรส การเลือกดูหนังใหม่ๆไม่ใช่เรื่องยากอีกต่อไป เพียงแต่ไปที่ช่องค้นหา สามารถพิมพ์ชื่อเรื่องหรือปีที่ฉาย ก็จะแสดงผลลัพธ์ลัพท์หนังที่อยากได้อย่างเร็ว
สำหรับคอหนังที่ถูกใจดูหนังดังบน Netflix ทางเว็บดูหนังออนไลน์ของพวกเราก็อัพเดทมาให้แก่คุณได้ดูหนังออนไลน์อย่างรวดเร็ว ท่านไม่มีความจำเป็นที่จะต้องเสียค่าใช้จ่ายสำหรับการสมัครสมาชิกรายเดือนอีกต่อไป ทางเว็บไซต์ของพวกเรานำหนังใหม่ชนโรง รวมทั้งหนังตามที่มีคุณภาพมาให้ทุกท่านได้รับดูกัน พูดได้ว่าไม่แพ้หนังบนเน็ตฟริกซ์อย่างยิ่งจริงๆ แล้วก็ที่สำคัญเรามีหนังให้เลือกรับดูมากกว่าไม่ว่าจะในกระแส หรือนอกกระแส เชื่อว่าทุกคนจะได้รับความสนุกสนานแบบเต็มอิ่ม ครบทุกอรรถรส การเลือกดูหนังใหม่ๆไม่ใช่เรื่องยากอีกต่อไป เพียงแต่ไปที่ช่องค้นหา สามารถพิมพ์ชื่อเรื่องหรือปีที่ฉาย ก็จะแสดงผลลัพธ์ลัพท์หนังที่อยากได้อย่างเร็ว

 m.jinda55 เว็บสล็อต เว็บไซต์ตรง มั่นคง ไม่มีอันตราย 100 มาลองลงทะเบียนเป็นสมาชิกแล้วไม่จำเป็นที่จะต้องมาวิตกกังวลหรือกลุ้มใจอะไรเลย ที่จะเพิ่มหนทางได้กำไรได้เป็นอย่างดี แล้วอยากชี้นำให้ทดสอบเข้ามาศึกษาถึงทางที่มองหาเว็บตรงที่มีความปลอดภัย,
m.jinda55 เว็บสล็อต เว็บไซต์ตรง มั่นคง ไม่มีอันตราย 100 มาลองลงทะเบียนเป็นสมาชิกแล้วไม่จำเป็นที่จะต้องมาวิตกกังวลหรือกลุ้มใจอะไรเลย ที่จะเพิ่มหนทางได้กำไรได้เป็นอย่างดี แล้วอยากชี้นำให้ทดสอบเข้ามาศึกษาถึงทางที่มองหาเว็บตรงที่มีความปลอดภัย,  เล่นสล็อตเว็บตรงได้เงินจริง มือใหม่ ปั๊มรายได้ไม่ยาก slot เครดิตฟรี
เล่นสล็อตเว็บตรงได้เงินจริง มือใหม่ ปั๊มรายได้ไม่ยาก slot เครดิตฟรี ขอขอบคุณweb
ขอขอบคุณweb 



 pg slot เว็บตรง สล็อตเล่นฟรีพลาดไม่ได้ สล็อต PG เว็บตรง แตกหนัก
pg slot เว็บตรง สล็อตเล่นฟรีพลาดไม่ได้ สล็อต PG เว็บตรง แตกหนัก

 ระบบ ทดสอบเล่นสล็อต PG เว็บไซต์ตรง เปิดให้บริการแล้วในประเทศไทย!
ระบบ ทดสอบเล่นสล็อต PG เว็บไซต์ตรง เปิดให้บริการแล้วในประเทศไทย! ระบบ ทดลองเล่นpg ของพวกเรามีดียังไงบ้าง?
ระบบ ทดลองเล่นpg ของพวกเรามีดียังไงบ้าง?
 ถ้าคุณกำลังมองหาเว็บสล็อตที่น่าไว้วางใจอยูเดี๋ยวนี้ เราขอแนะนำสล็อตเว็บของเรา ซึ่งเป็นสล็อตเว็บไซต์ตรงแตกง่ายได้เงินเร็ว ส่งตรงมาจากเว็บไซต์สล็อตหลักซึ่งเป็นเว็บแม่สล็อตที่น่าเชื่อ ได้รับการการันตีเรื่องประสิทธิภาพของเกมรวมถึงระบบการจ่ายเงินที่มีความเที่ยงตรง โดยคุณจะได้รับเงินทันทีที่คุณปรารถนาถอน โดยมีเจ้าหน้าที่รอดูแลโอนเงินในระบบเข้าบัญชีคุณภายในระยะเวลาไม่ถึงหนึ่งนาที นอกเหนือจากนั้นเว็บเรายังมีกิจกรรมทดสอบเล่น
ถ้าคุณกำลังมองหาเว็บสล็อตที่น่าไว้วางใจอยูเดี๋ยวนี้ เราขอแนะนำสล็อตเว็บของเรา ซึ่งเป็นสล็อตเว็บไซต์ตรงแตกง่ายได้เงินเร็ว ส่งตรงมาจากเว็บไซต์สล็อตหลักซึ่งเป็นเว็บแม่สล็อตที่น่าเชื่อ ได้รับการการันตีเรื่องประสิทธิภาพของเกมรวมถึงระบบการจ่ายเงินที่มีความเที่ยงตรง โดยคุณจะได้รับเงินทันทีที่คุณปรารถนาถอน โดยมีเจ้าหน้าที่รอดูแลโอนเงินในระบบเข้าบัญชีคุณภายในระยะเวลาไม่ถึงหนึ่งนาที นอกเหนือจากนั้นเว็บเรายังมีกิจกรรมทดสอบเล่น
 แม้คุณกำลังมองหา pg slot เว็บไซต์ตรง อยู่ล่ะก็? เพราะเหตุไรไม่ดูมาทางนี้ล่ะขอรับ ที่
แม้คุณกำลังมองหา pg slot เว็บไซต์ตรง อยู่ล่ะก็? เพราะเหตุไรไม่ดูมาทางนี้ล่ะขอรับ ที่ 
 ปากทางเข้า pg ทางเข้า pg กรรมวิธีการเล่นเกมสล็อตออนไลน์
ปากทางเข้า pg ทางเข้า pg กรรมวิธีการเล่นเกมสล็อตออนไลน์

 เพราะเหตุไรนักแทงหวย
เพราะเหตุไรนักแทงหวย  เพราะเหตุไรถึงต้องมีสลากกินแบ่งออนไลน์ แทงหวยสด
เพราะเหตุไรถึงต้องมีสลากกินแบ่งออนไลน์ แทงหวยสด ขอขอบพระคุณเว็ปไซต์
ขอขอบพระคุณเว็ปไซต์ 

 Pgslot เว็บตรง กับ Casinoruby88 สล็อตเว็บตรง อยู่ร่วมกันแล้วมากกว่าคุ้ม บอกเลยว่า การรวมตัวของเราสองบริษัทนั้น ทำให้เปอร์เซ็นต์การแตกหนักในทุกการหมุน เพิ่มขึ้นเป็นอันมาก เพราะว่าพวกเรา Casinoruby88 เป็น สล็อตเว็บไซต์ตรง สล็อตแตกหนัก อยู่แล้ว เพียงพอมาพบกับ pgslot เว็บตรง ซึ่งเป็นค่าย สล็อตแตกหนัก เช่นเดียวกัน ทำให้ยิ่งเราอยู่รวมกัน ยิ่งรวย! บอกเลยว่า การรวมตัวกันของพวกเรา สร้างแรงกระเพื่อมอย่างมหาศาลในตอนต้นปีให้หลัง ทำให้เหล่านักลงทุนทั่วโลกสังเกตกันตาเป็นมันอย่างยิ่งจริงๆจ้า ไม่เชื่ออย่าดูถูกดูแคลน สล็อตพีจี + Casinoruby88 นี่แหละของจริง มาทดลองกันได้เลยค่ะ!
Pgslot เว็บตรง กับ Casinoruby88 สล็อตเว็บตรง อยู่ร่วมกันแล้วมากกว่าคุ้ม บอกเลยว่า การรวมตัวของเราสองบริษัทนั้น ทำให้เปอร์เซ็นต์การแตกหนักในทุกการหมุน เพิ่มขึ้นเป็นอันมาก เพราะว่าพวกเรา Casinoruby88 เป็น สล็อตเว็บไซต์ตรง สล็อตแตกหนัก อยู่แล้ว เพียงพอมาพบกับ pgslot เว็บตรง ซึ่งเป็นค่าย สล็อตแตกหนัก เช่นเดียวกัน ทำให้ยิ่งเราอยู่รวมกัน ยิ่งรวย! บอกเลยว่า การรวมตัวกันของพวกเรา สร้างแรงกระเพื่อมอย่างมหาศาลในตอนต้นปีให้หลัง ทำให้เหล่านักลงทุนทั่วโลกสังเกตกันตาเป็นมันอย่างยิ่งจริงๆจ้า ไม่เชื่ออย่าดูถูกดูแคลน สล็อตพีจี + Casinoruby88 นี่แหละของจริง มาทดลองกันได้เลยค่ะ! Casinoruby88 ภูมิใจพรีเซ็นท์ ยอดเยี่ยมเทคนิคการปั่นสล็อต สำหรับ สล็อตเว็บไซต์ตรง ที่เป็นที่ชื่นชอบในเหล่าเซียนสล็อตกันอย่างล้นหลามมาตลอดตั้งแต่มีสล็อตออนไลน์มาจนถึงเดี๋ยวนี้ มาเริ่มกันดีกว่า ลุย!
Casinoruby88 ภูมิใจพรีเซ็นท์ ยอดเยี่ยมเทคนิคการปั่นสล็อต สำหรับ สล็อตเว็บไซต์ตรง ที่เป็นที่ชื่นชอบในเหล่าเซียนสล็อตกันอย่างล้นหลามมาตลอดตั้งแต่มีสล็อตออนไลน์มาจนถึงเดี๋ยวนี้ มาเริ่มกันดีกว่า ลุย!
 ทางเข้าสล็อตเว็บไซต์ตรง รวมเครดิตฟรี โบนัสเครดิตฟรี ไม่ต้องฝาก ไม่ต้องแชร์ เพียงแค่สมัครเป็นสมาชิก ถอนออกได้ นับว่าเป็นโบนัสแรกเข้าที่บรรดา slot เครดิตฟรี คาสิโนออนไลน์เครดิตฟรี ใช้จูงใจผู้เล่นใหม่ให้ลงทะเบียนสมัครสมาชิก โดยสมาชิกใหม่ทุกคนที่สมัครเข้ามาในขณะที่กำหนด จะได้รับ slot เครดิตฟรีเว็บตรง ไปทดลองวางเดิมพันแบบไม่เสียค่าใช้จ่ายใดๆก็ตามแลกเปลี่ยนมาด้วยข้อแม้ที่เว็บไซต์ระบุเอาไว้ เอ่ยมาแบบนี้ฟังดูดีใช่ไหมครับ
ทางเข้าสล็อตเว็บไซต์ตรง รวมเครดิตฟรี โบนัสเครดิตฟรี ไม่ต้องฝาก ไม่ต้องแชร์ เพียงแค่สมัครเป็นสมาชิก ถอนออกได้ นับว่าเป็นโบนัสแรกเข้าที่บรรดา slot เครดิตฟรี คาสิโนออนไลน์เครดิตฟรี ใช้จูงใจผู้เล่นใหม่ให้ลงทะเบียนสมัครสมาชิก โดยสมาชิกใหม่ทุกคนที่สมัครเข้ามาในขณะที่กำหนด จะได้รับ slot เครดิตฟรีเว็บตรง ไปทดลองวางเดิมพันแบบไม่เสียค่าใช้จ่ายใดๆก็ตามแลกเปลี่ยนมาด้วยข้อแม้ที่เว็บไซต์ระบุเอาไว้ เอ่ยมาแบบนี้ฟังดูดีใช่ไหมครับ
 สล็อตเล่นผ่านเครื่องใช้ไม้สอยไหนดีที่สุด
สล็อตเล่นผ่านเครื่องใช้ไม้สอยไหนดีที่สุด สล็อตเล่นผ่านเครื่องไม้เครื่องมือไหนเหมาะสมที่สุด บันเทิงใจได้ 24 ชม. เพิ่มผลกำไรได้ทั้งวัน
สล็อตเล่นผ่านเครื่องไม้เครื่องมือไหนเหมาะสมที่สุด บันเทิงใจได้ 24 ชม. เพิ่มผลกำไรได้ทั้งวัน
 แม้ทุกคนเคยมีความรู้สึกว่า ดูหนังผ่านอินเตอร์เน็ต หนังใหม่ ดูหนังผ่านเน็ต 2023 ดูตรงไหนก็เหมือนกัน คุณกำลังคิดผิดอย่างร้ายแรง มาดูกันดีกว่าว่ามันเว็บ ดูหนังผ่านอินเตอร์เน็ต หนังใหม่ ดูหนังผ่านอินเตอร์เน็ต 2023 มันต่างกันยังไงบ้าง?
แม้ทุกคนเคยมีความรู้สึกว่า ดูหนังผ่านอินเตอร์เน็ต หนังใหม่ ดูหนังผ่านเน็ต 2023 ดูตรงไหนก็เหมือนกัน คุณกำลังคิดผิดอย่างร้ายแรง มาดูกันดีกว่าว่ามันเว็บ ดูหนังผ่านอินเตอร์เน็ต หนังใหม่ ดูหนังผ่านอินเตอร์เน็ต 2023 มันต่างกันยังไงบ้าง? อัพเดทข่าวสารวงการหนัง
อัพเดทข่าวสารวงการหนัง 



 4 เหตุผลที่สล็อตออนไลน์คือเกมที่ฮิตที่สุดในโลก
4 เหตุผลที่สล็อตออนไลน์คือเกมที่ฮิตที่สุดในโลก

 สล็อต888 สล็อต แตกหนัก จัดเต็ม เว็บตรงฝากถอนไม่มีอย่างต่ำสล็อต888 เว็บ ไทยสล็อต 888 สล็อตเว็บตรงไม่ผ่านเอเย่นต์ สล็อต pg ที่ดีเยี่ยมที่สุด ในประเทศไทย เนื่องจากว่า สล็อต888 ของเรา เป็นหนึ่งในเว็บไซต์ สล็อตแตกหนัก ชั้นนำของประเทศไทยอย่างแน่แท้ในช่วงเวลานี้ เพราะด้วยความโด่งดังที่พวกเราสร้างมาอย่างนานจากการเป็นสล็อตเว็บไซต์ตรงในต่างแดน อย่างที่ทุกคนรู้กันว่า สล็อตเว็บตรงไม่ผ่านเอเย่นต์ ในประเทศไทยมันหายากมาก พวกเราจึงขยายเข้ามาเปิด ไทยสล็อต 888 ในประเทศไทย สล็อต888 เป็น เว็บไซต์สล็อตที่มีชื่อเป็นวงกว้างในต่างแดน เนื่องจากด้วยมาตรฐานที่สูงของคาสิโน ทำให้มีลูกค้ามาใช้บริการกันอย่างมากมาย เป็น สล็อตเว็บตรงไม่ผ่านเอเย่นต์ เว็บไซต์ตรงฝากถอนไม่มีขั้นต่ำ และท้ายที่สุด เราก็มาถึงเมืองไทย บอกเลยว่าพวกเรา สล็อต888 ไม่ใช่ผู้แทน แต่เราเป็นตัวจริงเสียงจริง ไม่ใช่ผู้แสดงเหมือนเว็บไซต์อื่นๆโดยเหตุนี้ ไทยสล็อต 888 เป็น สล็อตเว็บไซต์ตรงไม่ผ่านเอเย่นต์ และก็ยังเป็น สล็อต pg ที่เหมาะสมที่สุด อย่างแท้จริง
สล็อต888 สล็อต แตกหนัก จัดเต็ม เว็บตรงฝากถอนไม่มีอย่างต่ำสล็อต888 เว็บ ไทยสล็อต 888 สล็อตเว็บตรงไม่ผ่านเอเย่นต์ สล็อต pg ที่ดีเยี่ยมที่สุด ในประเทศไทย เนื่องจากว่า สล็อต888 ของเรา เป็นหนึ่งในเว็บไซต์ สล็อตแตกหนัก ชั้นนำของประเทศไทยอย่างแน่แท้ในช่วงเวลานี้ เพราะด้วยความโด่งดังที่พวกเราสร้างมาอย่างนานจากการเป็นสล็อตเว็บไซต์ตรงในต่างแดน อย่างที่ทุกคนรู้กันว่า สล็อตเว็บตรงไม่ผ่านเอเย่นต์ ในประเทศไทยมันหายากมาก พวกเราจึงขยายเข้ามาเปิด ไทยสล็อต 888 ในประเทศไทย สล็อต888 เป็น เว็บไซต์สล็อตที่มีชื่อเป็นวงกว้างในต่างแดน เนื่องจากด้วยมาตรฐานที่สูงของคาสิโน ทำให้มีลูกค้ามาใช้บริการกันอย่างมากมาย เป็น สล็อตเว็บตรงไม่ผ่านเอเย่นต์ เว็บไซต์ตรงฝากถอนไม่มีขั้นต่ำ และท้ายที่สุด เราก็มาถึงเมืองไทย บอกเลยว่าพวกเรา สล็อต888 ไม่ใช่ผู้แทน แต่เราเป็นตัวจริงเสียงจริง ไม่ใช่ผู้แสดงเหมือนเว็บไซต์อื่นๆโดยเหตุนี้ ไทยสล็อต 888 เป็น สล็อตเว็บไซต์ตรงไม่ผ่านเอเย่นต์ และก็ยังเป็น สล็อต pg ที่เหมาะสมที่สุด อย่างแท้จริง ขอขอบคุณอ้างอิง
ขอขอบคุณอ้างอิง 
 ขอขอบคุณมากที่มา
ขอขอบคุณมากที่มา 



 บาคาร่า168 บาคาร่า168 เว็บไซต์คาสิโนออนไลน์เป็นอีกหนึ่งทางออกการทำเงินสร้างรายได้ นับว่าเป็นเว็บสื่อกลางของต้นตอเดิมพันและก็นักเสี่ยงโชค นักพนันสามารถเล่น
บาคาร่า168 บาคาร่า168 เว็บไซต์คาสิโนออนไลน์เป็นอีกหนึ่งทางออกการทำเงินสร้างรายได้ นับว่าเป็นเว็บสื่อกลางของต้นตอเดิมพันและก็นักเสี่ยงโชค นักพนันสามารถเล่น ลงทะเบียนสมัครสมาชิกใหม่กันทั้งที จะไม่มีโปรโมชั่นให้ได้อย่างไรล่ะครับจริงไหม! มาครับผม joker123 จัดให้แบบสมใจ สมัครเป็นสมาชิกใหม่วันนี้ รับโปรโมชั่นสมาชิกใหม่ รับโบนัสเครดิตฟรีกันไปเลย ถ้าคุณฝากเงิน 100 บาท รับ 200 บาท,
ลงทะเบียนสมัครสมาชิกใหม่กันทั้งที จะไม่มีโปรโมชั่นให้ได้อย่างไรล่ะครับจริงไหม! มาครับผม joker123 จัดให้แบบสมใจ สมัครเป็นสมาชิกใหม่วันนี้ รับโปรโมชั่นสมาชิกใหม่ รับโบนัสเครดิตฟรีกันไปเลย ถ้าคุณฝากเงิน 100 บาท รับ 200 บาท, 


 ค่ายดังทุกค่าย โจ๊กเกอร์123 joker123th.co.in 22 มิถุนา 2567 Delmar อยากรวยต้องลอง โจ๊กเกอร์123ปรึกษาฟรี Top 24
ค่ายดังทุกค่าย โจ๊กเกอร์123 joker123th.co.in 22 มิถุนา 2567 Delmar อยากรวยต้องลอง โจ๊กเกอร์123ปรึกษาฟรี Top 24

 สล็อตเว็บตรง ทดลองเล่นสล็อตทุนฟรี เล่นได้แต่ละวันตลอด 24 ชั่วโมง
สล็อตเว็บตรง ทดลองเล่นสล็อตทุนฟรี เล่นได้แต่ละวันตลอด 24 ชั่วโมง ในช่วงปัจจุบันการเล่น slot ก็ได้รับความนิยมอย่างยิ่ง ซึ่งมีเว็บไซต์หลายแห่งที่เน้นให้บริการสล็อตเว็บไซต์ตรง ที่ทำให้ผู้เล่นสามารถเข้าเล่นได้โดยตรงโดยไม่ต้องผ่านเอเย่นต์ เอ่ยถึงเรื่องของสล็อตเว็บไซต์ตรงที่มีการทดลองเล่นslot99ทุนฟรีและสามารถเล่นได้วันแล้ววันเล่าตลอด 1 วัน การให้บริการทดสอบเล่นใน
ในช่วงปัจจุบันการเล่น slot ก็ได้รับความนิยมอย่างยิ่ง ซึ่งมีเว็บไซต์หลายแห่งที่เน้นให้บริการสล็อตเว็บไซต์ตรง ที่ทำให้ผู้เล่นสามารถเข้าเล่นได้โดยตรงโดยไม่ต้องผ่านเอเย่นต์ เอ่ยถึงเรื่องของสล็อตเว็บไซต์ตรงที่มีการทดลองเล่นslot99ทุนฟรีและสามารถเล่นได้วันแล้ววันเล่าตลอด 1 วัน การให้บริการทดสอบเล่นใน  การทดลองเล่นslot99ทุนฟรีช่วยทำให้ผู้เล่นได้รับความรู้รวมทั้งความเข้าใจเกี่ยวกับกฎของเกม รวมทั้งการทดลองกระบวนการเล่นแล้วก็กระบวนการชนะเงินรางวัล ผู้เล่นสามารถใช้โอกาสที่ทดลองเล่นสล็อตทุนฟรีเพื่อปรับปรุงแก้ไขวิธีการเล่น รวมทั้งการทดลองสร้างแผนการใหม่ๆเพื่อเพิ่มจังหวะสำหรับในการชนะเงินรางวัล การทดลองเล่นslotทุนฟรียังช่วยให้ผู้เล่นสามารถเพลิดเพลินเจริญใจกับการเล่นโดยไม่จำเป็นที่จะต้องมาวิตกกังวลหรือกลุ้มใจหัวข้อการสูญเสียเงิน ทำให้มีความสนุกสนานรวมทั้งติดใจกับเกมสล็อตมากยิ่งขึ้น
การทดลองเล่นslot99ทุนฟรีช่วยทำให้ผู้เล่นได้รับความรู้รวมทั้งความเข้าใจเกี่ยวกับกฎของเกม รวมทั้งการทดลองกระบวนการเล่นแล้วก็กระบวนการชนะเงินรางวัล ผู้เล่นสามารถใช้โอกาสที่ทดลองเล่นสล็อตทุนฟรีเพื่อปรับปรุงแก้ไขวิธีการเล่น รวมทั้งการทดลองสร้างแผนการใหม่ๆเพื่อเพิ่มจังหวะสำหรับในการชนะเงินรางวัล การทดลองเล่นslotทุนฟรียังช่วยให้ผู้เล่นสามารถเพลิดเพลินเจริญใจกับการเล่นโดยไม่จำเป็นที่จะต้องมาวิตกกังวลหรือกลุ้มใจหัวข้อการสูญเสียเงิน ทำให้มีความสนุกสนานรวมทั้งติดใจกับเกมสล็อตมากยิ่งขึ้น
 cafe444 เข้าเล่น pg slot เว็บตรง ไม่ผ่านเอเย่นต์ ปัจจุบัน สมัครฟรี
cafe444 เข้าเล่น pg slot เว็บตรง ไม่ผ่านเอเย่นต์ ปัจจุบัน สมัครฟรี การฝากถอนเงินโดยไม่มีขั้นต่ำมีข้อดีในเรื่องของความยืดหยุ่น เนื่องจากว่าผู้เล่นสามารถทำธุรกรรมได้ตามต้องการของตน ไม่จำเป็นที่จะต้องระบุจำนวนเงินขั้นต่ำที่จำต้องถอนหรือฝาก เป็นต้นว่า หากผู้เล่นมีเงินเกินกำหนดที่อยากถอนออกมา ไม่จำเป็นที่ต้องรอคอยตราบจนกระทั่งจะมีจำนวนเงินที่ตรงตามขั้นต่ำที่เว็บไซต์ระบุ
การฝากถอนเงินโดยไม่มีขั้นต่ำมีข้อดีในเรื่องของความยืดหยุ่น เนื่องจากว่าผู้เล่นสามารถทำธุรกรรมได้ตามต้องการของตน ไม่จำเป็นที่จะต้องระบุจำนวนเงินขั้นต่ำที่จำต้องถอนหรือฝาก เป็นต้นว่า หากผู้เล่นมีเงินเกินกำหนดที่อยากถอนออกมา ไม่จำเป็นที่ต้องรอคอยตราบจนกระทั่งจะมีจำนวนเงินที่ตรงตามขั้นต่ำที่เว็บไซต์ระบุ

 • เปิดให้บริการตลอด 24 ชั่วโมง ถ้าหากคุณเป็นอีกผู้ที่ประทับใจการเล่นเกมpunpro777 แต่ว่ามิได้สะดวกจะเล่นในเวลาปกติทั่วๆไป คุณอาจจะชอบเล่นตอนเช้ามากๆหรือเวลาดึกมากๆก็ไม่จำเป็นที่จะต้องมาวิตกกังวลจิตใจไปขอรับ เพราะเราเปิดให้บริการตลอด 24 ชั่วโมงอยู่แล้ว เล่นpgslotสนุกได้แต่ละวัน ไม่มีทางหยุดแน่นอนขอรับ ลองเลย!
• เปิดให้บริการตลอด 24 ชั่วโมง ถ้าหากคุณเป็นอีกผู้ที่ประทับใจการเล่นเกมpunpro777 แต่ว่ามิได้สะดวกจะเล่นในเวลาปกติทั่วๆไป คุณอาจจะชอบเล่นตอนเช้ามากๆหรือเวลาดึกมากๆก็ไม่จำเป็นที่จะต้องมาวิตกกังวลจิตใจไปขอรับ เพราะเราเปิดให้บริการตลอด 24 ชั่วโมงอยู่แล้ว เล่นpgslotสนุกได้แต่ละวัน ไม่มีทางหยุดแน่นอนขอรับ ลองเลย! โปรโมชั่นฝากแรกของวัน ฝากรับโบนัสเครดิตฟรีจาก PUNPRO777 ได้เลยโดยทันที 20% มารับกันได้เลย!
โปรโมชั่นฝากแรกของวัน ฝากรับโบนัสเครดิตฟรีจาก PUNPRO777 ได้เลยโดยทันที 20% มารับกันได้เลย!
 2. โปรโมชั่น โบนัสปันสุข ฝาก 88 บาท รับ 188 บาท ทำยอด 5 เท่า ถอนได้ 200 บาท รับได้วันละ 1 ครั้ง เล่นได้เฉพาะเกมสล็อต พร้อมเสนอแนะ 4 เกมดังพร้อมลุยบนทางสล็อตเงินล้าน
2. โปรโมชั่น โบนัสปันสุข ฝาก 88 บาท รับ 188 บาท ทำยอด 5 เท่า ถอนได้ 200 บาท รับได้วันละ 1 ครั้ง เล่นได้เฉพาะเกมสล็อต พร้อมเสนอแนะ 4 เกมดังพร้อมลุยบนทางสล็อตเงินล้าน 3. โปรโมชั่น โปรเด็ดจำเป็นต้องโดน ฝาก 49 บาท รับ 100 บาท ทำยอด 600 บาท ถอนได้ 200 บาท ทำยอด 600 บาท ถอนได้ 200 บาท รับได้วันละ 1 ครั้งเพียงแค่นั้น เล่นได้เฉพาะสล็อตรวมทั้งห้ามซื้อฟรีสปินเด็ดขาด!
3. โปรโมชั่น โปรเด็ดจำเป็นต้องโดน ฝาก 49 บาท รับ 100 บาท ทำยอด 600 บาท ถอนได้ 200 บาท ทำยอด 600 บาท ถอนได้ 200 บาท รับได้วันละ 1 ครั้งเพียงแค่นั้น เล่นได้เฉพาะสล็อตรวมทั้งห้ามซื้อฟรีสปินเด็ดขาด!
 Slotxo24hr เราเป็นคนใดมาจากไหน? เพราะอะไรเราถึงน่าเล่นกว่าที่อื่น? วันนี้เรามีคำตอบ!
Slotxo24hr เราเป็นคนใดมาจากไหน? เพราะอะไรเราถึงน่าเล่นกว่าที่อื่น? วันนี้เรามีคำตอบ! เหตุผลโน่นก็เนื่องจาก ในตอนแรก กลุ่มของเรา สล็อตxo วางแผนไว้ว่าควรเปิดเว็บไซต์ปากทางเข้าสล็อตออนไลน์ ที่ใดกันดี แล้วบทสรุปออกมาเป็นโซนเอเชีย และก็ที่เลือกไทยเป็นประเทศเป็นประเทศแรกเลย นั่นก็เพราะ
เหตุผลโน่นก็เนื่องจาก ในตอนแรก กลุ่มของเรา สล็อตxo วางแผนไว้ว่าควรเปิดเว็บไซต์ปากทางเข้าสล็อตออนไลน์ ที่ใดกันดี แล้วบทสรุปออกมาเป็นโซนเอเชีย และก็ที่เลือกไทยเป็นประเทศเป็นประเทศแรกเลย นั่นก็เพราะ ขอขอบคุณมากby web
ขอขอบคุณมากby web 
 Fullslotpg สล็อตเว็บตรงไม่ผ่านเอเย่นต์ เว็บไซต์ตรงแท้ 100% API มาตรฐานระดับนานาชาติ สมัครเลย!
Fullslotpg สล็อตเว็บตรงไม่ผ่านเอเย่นต์ เว็บไซต์ตรงแท้ 100% API มาตรฐานระดับนานาชาติ สมัครเลย! เล่นสล็อตออนไลน์กับ fullslotpg ได้รับสิทธิพิเศษแบบจัดเต็มที่ไม่ซ้ำใคร มาลองกัน!
เล่นสล็อตออนไลน์กับ fullslotpg ได้รับสิทธิพิเศษแบบจัดเต็มที่ไม่ซ้ำใคร มาลองกัน!

 ขอขอบพระคุณเว็บ
ขอขอบพระคุณเว็บ  เจอกับความสนุกและความเพลิดเพลินจัดเต็มจาก PUNPRO777 เว็บตรงมาตรฐานสุดยอด สมัครเลย!
เจอกับความสนุกและความเพลิดเพลินจัดเต็มจาก PUNPRO777 เว็บตรงมาตรฐานสุดยอด สมัครเลย! เพียงแค่ทำเงินจากการเล่น
เพียงแค่ทำเงินจากการเล่น เล่นเสียมีคืนจาก PUNPRO กันไปเลย! กับโปรโมชั่น Cash Back 40% รับสูงสุด 5,000 บาทในทันที!
เล่นเสียมีคืนจาก PUNPRO กันไปเลย! กับโปรโมชั่น Cash Back 40% รับสูงสุด 5,000 บาทในทันที! เกมดีบอกต่อกับ PGSLOT ขอพาทุกคนไปสู่ดินแดนแห่งความฝันกับ Dream of Macao จะเป็นอย่างไรมาดูกัน!
เกมดีบอกต่อกับ PGSLOT ขอพาทุกคนไปสู่ดินแดนแห่งความฝันกับ Dream of Macao จะเป็นอย่างไรมาดูกัน! มองหาทางเข้าPG มองหาเรา slot เว็บไซต์ตรงไม่ผ่านเอเย่นต์ เว็บตรงแท้ 100% พร้อมบริการแล้ว สมัครเลย!
มองหาทางเข้าPG มองหาเรา slot เว็บไซต์ตรงไม่ผ่านเอเย่นต์ เว็บตรงแท้ 100% พร้อมบริการแล้ว สมัครเลย! แนวทางพิชิตเกมสล็อตออนไลน์แบบง่ายๆสไตล์ pg slot ที่ไม่ว่าใครก็สามารถทำเป็นแน่ๆ
แนวทางพิชิตเกมสล็อตออนไลน์แบบง่ายๆสไตล์ pg slot ที่ไม่ว่าใครก็สามารถทำเป็นแน่ๆ
